
Higher and foundation tiers
The Greeks philosopher Democritus suggested over 2500 years ago that matter was made up
of solid tiny balls called atoms. Democritus thought of atoms
as unbreakable spheres;
in fact the word atom comes from the Greek word atomos which translates as - "can't be split".
However we know today that it is possible to split the atom into smaller pieces and that
atoms
are not solid object but are mostly made up of empty space. It was only in the 20th century following
the work of scientists such as J.J.Thomson, Ernest Rutherford and Niels Bohr
that scientists finally realised that atoms were made up of smaller particles and Democritus' idea of atoms as solid spheres was finally rejected.
J.J Thomson was responsible for discovered the electron in 1897. This discovery led the way for new ideas
on the internal structure of atoms and allowed the brilliant scientist Ernest Rutherford to discover both the
proton and the nucleus. Further work by Niels Bohr suggested that the electrons orbit the nucleus in shells
or rings. The work of these scientists and others led to a new model of the atom; the nuclear atom.
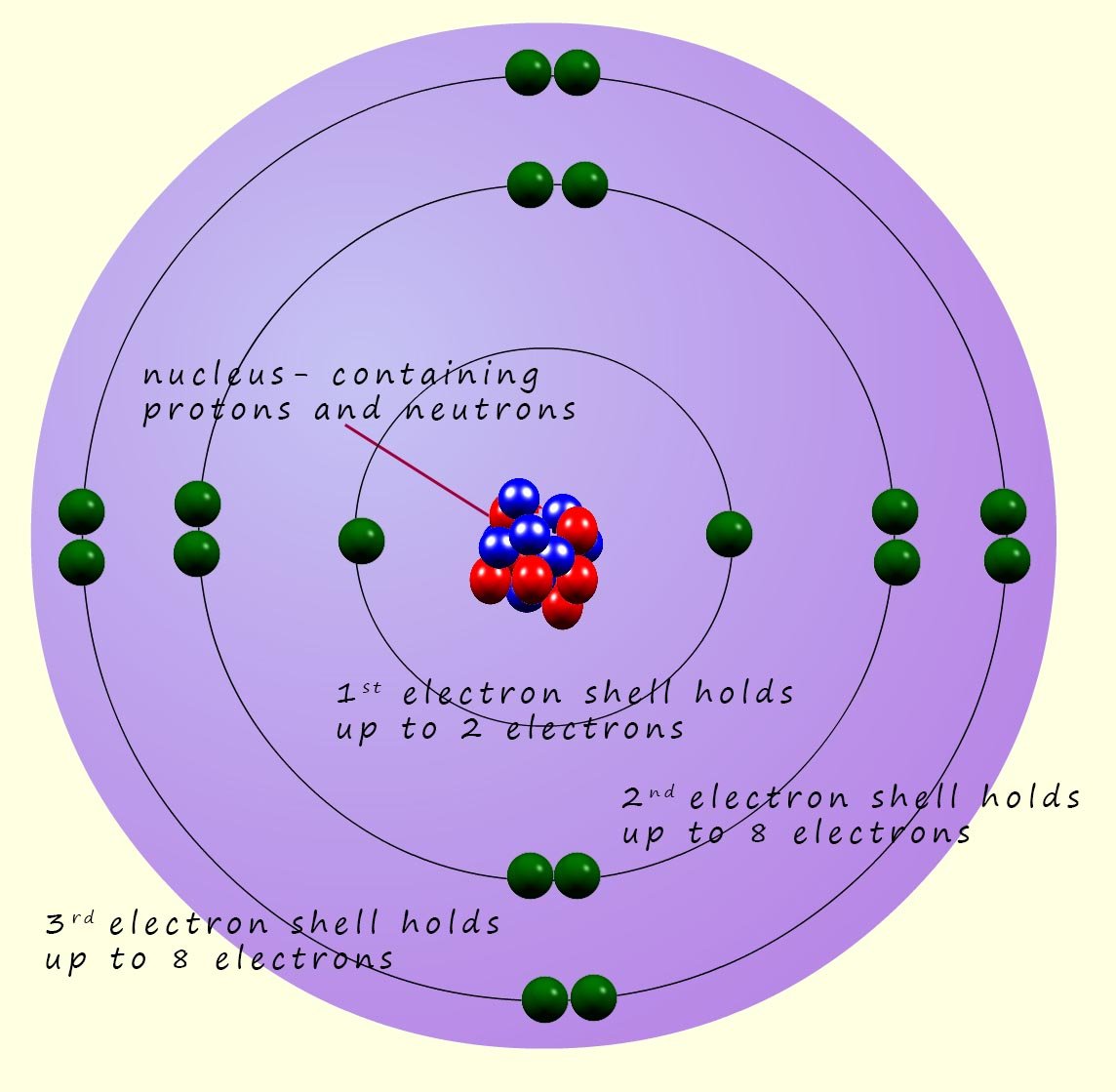
Atoms are very small! We might describe something small as microscopic but microscopic objects have sizes of about 1mm (0.001m). Atoms are much much smaller than this; Atoms may have a radius of around 1 x 10-10m (0.0000000001m) or 0.1nm ( 1 nanometre= 1 x 10-9m), about 1 million atoms stacked end to end would be about as thick as a human hair. The modern model we use to describe the internal structure of an atom is often called the Bohr atom after the Danish physicist Niels Bohr. Bohr suggested that the electrons in an atom orbit the nucleus in a series of shells or levels. The nucleus which was discovered by Ernest Rutherford, contains the protons and neutrons.
The table below gives some information on the mass and charge of the 3 sub-atomic particles found inside the atom. The protons and neutrons which are found in the nucleus are heavy particles and are responsible for almost all the mass of the atom. In comparison the mass of the electron is very small when compared to the masses of protons and neutrons. It takes almost 2000 electrons to make up the mass of a single neutron or proton, so for most practical purposes you can assume the mass of the electron is zero. This means that all the mass of the atom is concentrated inside the tiny nucleus. If you divide the mass of the proton by the mass of the neutron you get an answer of 1, this is why masses of the neutrons and protons are taken as 1. When compared to or relative to each other they are the same.
| particle | relative mass | actual mass in Kg | Relative charge | Where in the atom it is found | |
|---|---|---|---|---|---|
| proton | 1 | 1.67 x 10-27 | +1 | nucleus | |
| neutron | 1 | 1.67 x 10-27 | 0 | nucleus | |
| electron | 0 | 9.11 x 10-31 | -1 | electron shells or rings |
Test your understanding of atomic structure by completing the activity below. The words in the yellow boxes are used to fill in the empty boxes in the paragraph below, simply click the word in the yellow box and then click the blue box it needs to be placed in.